Commissioning - GMP Certification
Intro
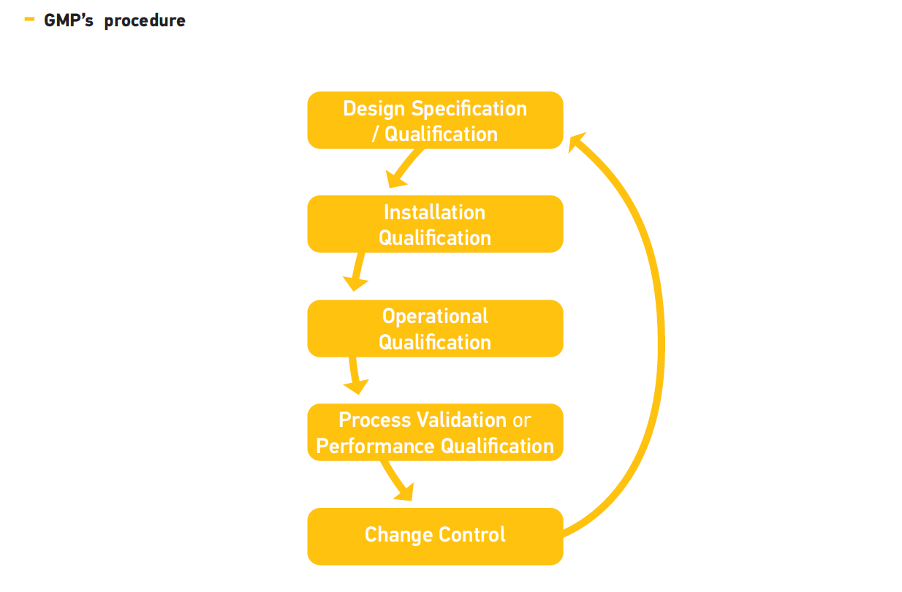
What is a GMP Certification
GMP is a system for ensuring that products and services are consistently produced, offered and controlled according to quality standards. The design and construction of cleanrooms must be based on EN ISO 14644 and GMP guidelines.
The basic principles and application of qualification and validation are described in Annex 15 of the PIC/S and EU Guide of GMP. A GMP certification reinforces the assertion that the project meets all the criteria to be fully operational and functional with the objective of providing the required quality level.
Briefly
Axis Medical, supplies and installs custom made cleanroom modular wall systems, according to the requirements and specifications of the project, combined with our architectural expertise.
Design Qualification (DQ)
- The first element of the validation of new facilities, systems or equipment is the design qualification (DQ)
- The compliance of the design with GMP should be demonstrated and documented
Installation Qualification (IQ)
It is performed on new or modified facilities, systems and equipment:
- Verification that equipment and material meet the design purpose
- Verification of the manufacturing process of equipment and materials
- Verification that equipment and material installation meet the industry standard of care
- Verification that the pre-start functions of the equipment are completed and according to manufacturer requirements
- Verification that equipment and system initial start-up meets established protocols
- Test and balance report
- Assembly of operation and maintenance manuals
Operational Qualification (OQ)
OQ includes but is not limited to the following:
(a) Tests that have been developed from knowledge of processes, systems and equipment
(b) Tests to include a condition or a set of conditions encompassing upper and lower operating limits, sometimes referred to as “worst case” conditions
(c) Clean room operational verification with the following major components:
- Clean room enclosure
- Lighting/electrical
- Noise
- Process equipment and systems
- HEPA filters
- HVAC systems
- Vibration
Performance Qualification (PQ)
- Performance qualification (PQ) follows the successful completion of the installation and operational qualification.
- PQ includes but is not limited to the following:
- Tests, using production materials, qualified substitutes or simulated products, that have been developed from knowledge of the process and the facilities, systems or equipment
- Tests to include a condition or set of conditions encompassing upper and lower operating limits
- Although PQ is described as a separate activity, it may in some cases be appropriate to perform it in conjunction with OQ